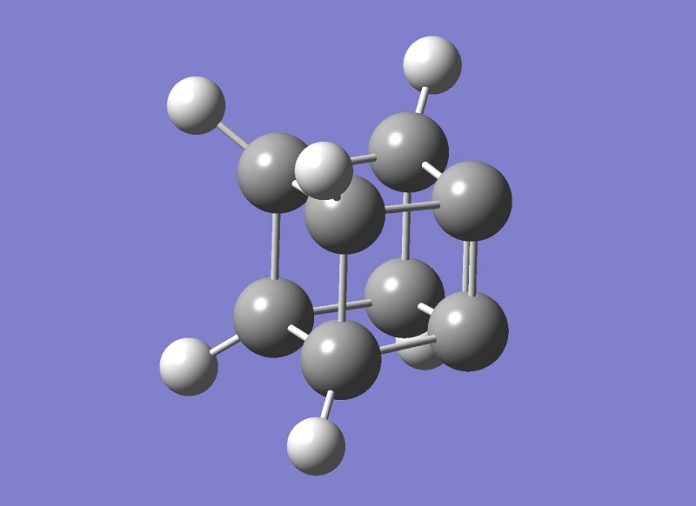
Dhealthwellness.com – Carbon is a very abundant element in our environment, and this fact is important to understand. Its chemical formula is C13H17O2. In simple terms, carbon is a compound with four carbon atoms, two of which are in the outer ring. The ring is planar, while double carbon bonds are tetrahedral. A triple bond means that there are two atoms on either side of the molecule.
Carbon Has Unique Properties
Unlike other elements, carbon has unique properties. It can form rings of five, six, and eight members. These rings are connected by single or double bonds, and nitrogen is a substitute for carbon. This makes carbon a very versatile element. Its many uses are almost limitless, and its abundance will continue to rise over time. However, it is essential to understand the properties of carbon, and how it is a constituent of other compounds.
Carbon molecules come in a variety of shapes, and the three classes of carbon compounds are hexagons and pentagons. These three structures can be easily understood through visualization, and a java applet allows you to explore their molecular structure in more detail. This interactive tool can be a useful tool when learning about the different types of molecules in the environment. The image can be rotated and can be used to learn about new materials.
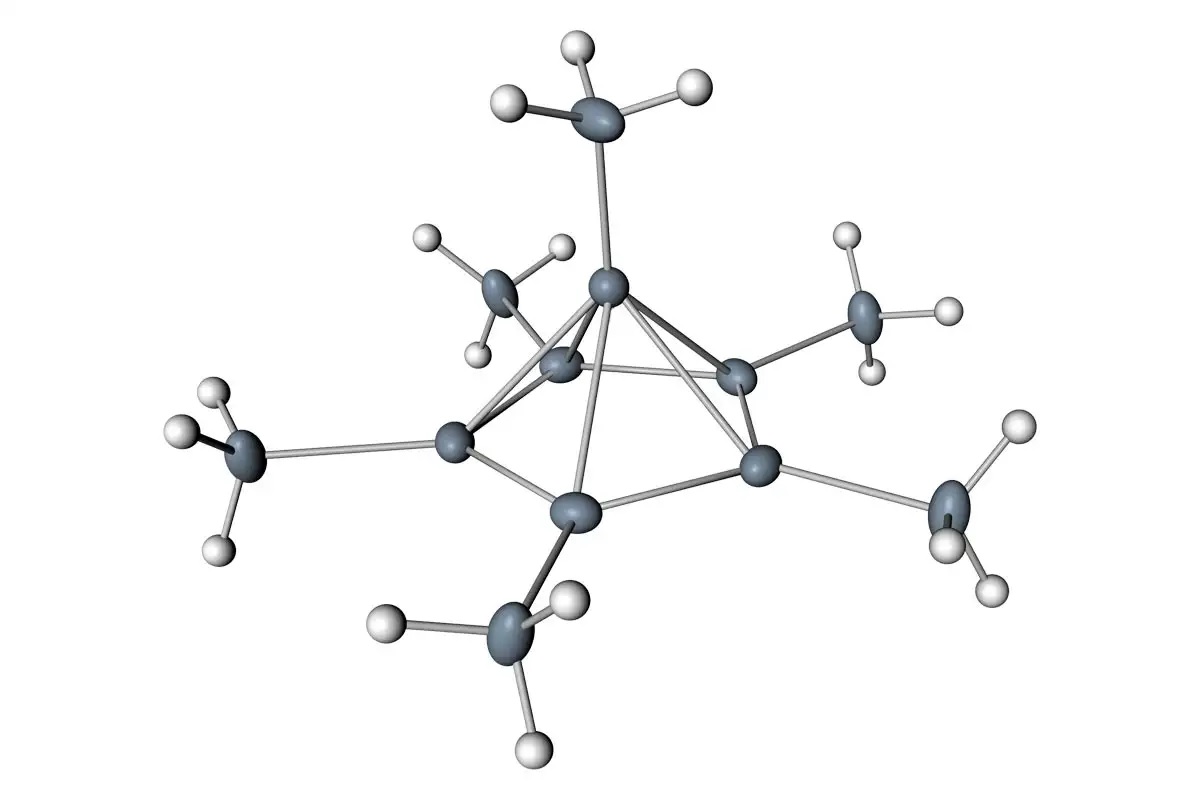
The three main c are fullerenes, nitriles, and benzenes. The former forms the icosahedron, whereas the latter is a seven-membered ring. Compared to the icosahedron, fullerenes have two hexagonal faces that are connected by single bonds. The second is the asymmetric fullerene, which is made of nitrile, while the last is a tetrahedral structure.
Complex Compound Mixtures
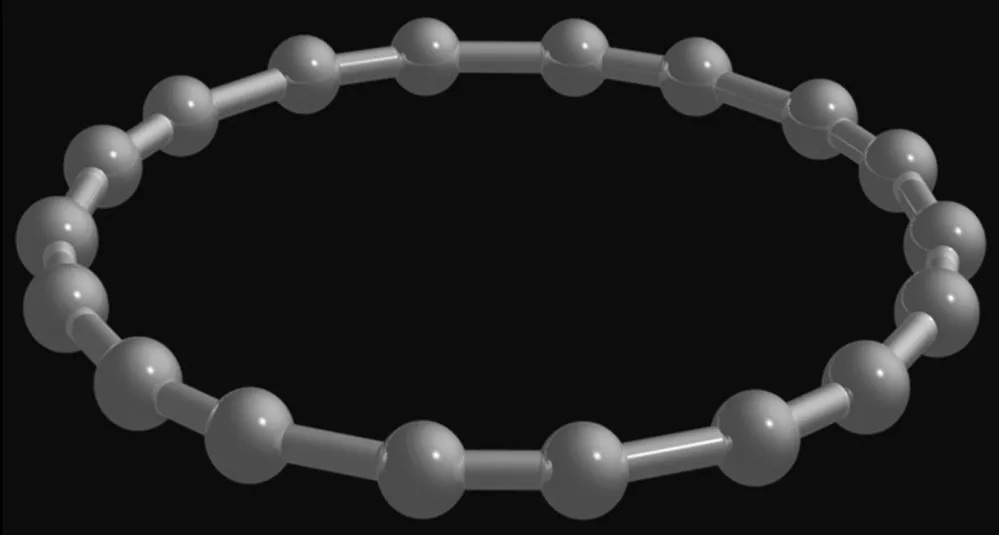
A carbon molecule is a compound of carbon atoms linked together. This is a complex mixture of compounds, ranging from the hard, clear diamonds to the soft, malleable graphite. The cycle carbon ring is an elegant and versatile compound. Scientists have been trying to create it for over a century but failed with previous attempts. It is too volatile to study in any serious way. Therefore, scientists are still pursuing it.
The third class of carbon molecules is fullerenes. This molecule has 60 carbon atoms and a double bond in the middle. It is composed of two carbon atoms, which form four covalent bonds. In addition, two other molecules with one-carbon molecule share a pair of electrons. In addition to its unique properties, carbon molecule is an excellent examples of cycle carbon molecule.
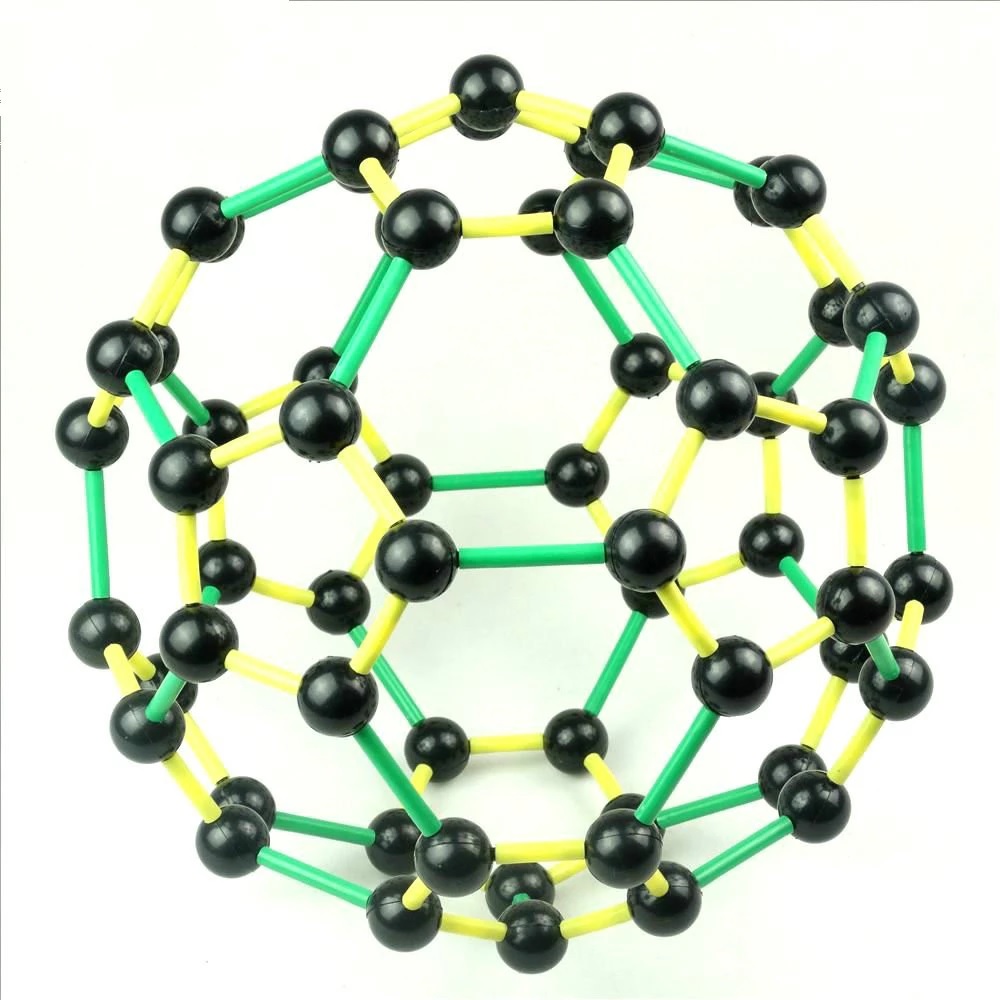
Fullerenes are a class of carbon compounds. They contain 60 carbons and resemble a tetrahedron. These compounds are very interesting because they have geometric structures. The icosahedron is made up of hexagons and pentagons. The cylinder has four carbon atoms, and a fullerene molecule has four. This structure is the basis of a ring of carbons.
Atoms Contain 60 Molecular Atoms
The second class of carbon molecules is fullerenes. These atoms contain 60 carbon atoms, and their geometric structure resembles that of the familiar icosahedron. For a better understanding of fullerenes, take a look at a java applet. Try rotating the image to see a fullerene. You will notice that it has three carbon atoms, one is an octahedron.
A ring is an organic compound. It contains two or more carbon atoms, and it is made up of two or more carbon atoms. The carbon ring in a ring has a double bond, which prevents free rotation around the ring. The octahedral group is another type of octahedroxycarbon. The octahedrene ring is the most common form of the octahedron.
The carbon atom is one of the most important elements in the universe, as it can be used to make many different compounds. Its molecular structure is what makes carbon so valuable, and it can bond with a variety of other elements to form an essential compound. The carbon atom is extremely small, so it can fit inside of a very large molecule. It is also a very important element in the environment, as it balances the chemical reactions in water and the atmosphere.
Reference: